Answer:
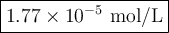
Step-by-step explanation:
Assume that you have mixed 135 mL of 0.0147 mol·L⁻¹ NiCl₂ with 190 mL of 0.250 mol·L⁻¹ NH₃.
1. Moles of Ni²⁺
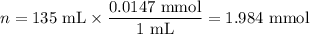
2. Moles of NH₃
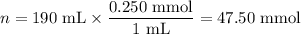
3. Initial concentrations after mixing
(a) Total volume
V = 135 mL + 190 mL = 325 mL
(b) [Ni²⁺]
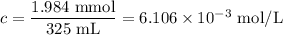
(c) [NH₃]
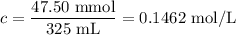
3. Equilibrium concentration of Ni²⁺
The reaction will reach the same equilibrium whether it approaches from the right or left.
Assume the reaction goes to completion.
Ni²⁺ + 6NH₃ ⇌ Ni(NH₃)₆²⁺
I/mol·L⁻¹: 6.106×10⁻³ 0.1462 0
C/mol·L⁻¹: -6.106×10⁻³ 0.1462-6×6.106×10⁻³ 0
E/mol·L⁻¹: 0 0.1095 6.106×10⁻³
Then we approach equilibrium from the right.
Ni²⁺ + 6NH₃ ⇌ Ni(NH₃)₆²⁺
I/mol·L⁻¹: 0 0.1095 6.106×10⁻³
C/mol·L⁻¹: +x +6x -x
E/mol·L⁻¹: x 0.1095+6x 6.106×10⁻³-x
![K_{\text{f}} = \frac{\text{[Ni(NH$_(3)$)$_(6)^(2+)$]}}{\text{[Ni$^(2+)$]}\text{[NH$_(3)$]}^(6)} = 2.0 * 10^(8)](https://img.qammunity.org/2021/formulas/chemistry/college/a00ktj4ev43sqcqpo05efteydu0hne7bfe.png)
Kf is large, so x ≪ 6.106×10⁻³. Then
![K_{\text{f}} = \frac{\text{[Ni(NH$_(3)$)$_(6)^(2+)$]}}{\text{[Ni$^(2+)$]}\text{[NH$_(3)$]}^(6)} = 2.0 * 10^(8)\\\\(6.106 * 10^(-3))/(x* 0.1095^(6)) = 2.0 * 10^(8)\\\\6.106 * 10^(-3) = 2.0 * 10^(8)* 0.1095^(6)x= 345.1x\\x= (6.106 * 10^(-3))/(345.1) = 1.77 * 10^(-5)\\\\\text{The concentration of Ni$^(2+)$ at equilibrium is $\large \boxed{\mathbf{1.77 * 10^(-5)}\textbf{ mol/L}}$}](https://img.qammunity.org/2021/formulas/chemistry/college/nufg8gsisnurnrak2gsy6z7eb1j4cf788g.png)