Step-by-step explanation:
It is given that,
Initial orbit of electrons,
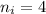
Final orbit of electrons,
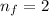
We need to find energy, wavelength and frequency of the wave.
When atom make transition from one orbit to another, the energy of wave is given by :
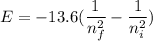
Putting all the values we get :
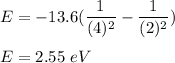
We know that :
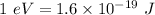
So,
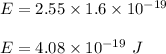
Energy of wave in terms of frequency is given by :
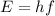
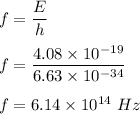
Also,
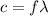
 is wavelength
is wavelength
So,
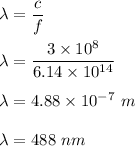
Hence, this is the required solution.