Answer:
The heat absorbed by the sample of water is 3,294.9 J
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
The sensible heat of a body is the amount of heat received or transferred by a body when it undergoes a temperature variation (Δt) without there being a change of physical state (solid, liquid or gaseous). Its mathematical expression is:
Q = c * m * ΔT
Where Q is the heat exchanged by a body of mass m, made up of a specific heat substance c and where ΔT is the temperature variation.
In this case:
- Q=?
- m= 45 g
- c= 4.184
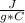
- ΔT= Tfinal - Tinitial= 38.5 C - 21 C= 17.5 C
Replacing:
Q= 4.184
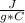 * 45 g* 17.5 C
* 45 g* 17.5 C
Solving:
Q=3,294.9 J
The heat absorbed by the sample of water is 3,294.9 J