Answer:
Approximately
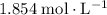 .
.
Step-by-step explanation:
Note that both figures in the question come with four significant figures. Therefore, the answer should also be rounded to four significant figures. Intermediate results should have more significant figures than that.
Formula mass of strontium hydroxide
Look up the relative atomic mass of
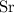 ,
,
 , and
, and
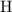 on a modern periodic table. Keep at least four significant figures in each of these atomic mass data.
on a modern periodic table. Keep at least four significant figures in each of these atomic mass data.
Calculate the formula mass of
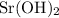 :
:
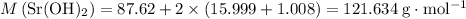 .
.
Number of moles of strontium hydroxide in the solution
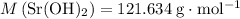 means that each mole of
means that each mole of
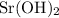 formula units have a mass of
formula units have a mass of
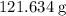 .
.
The question states that there are
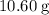 of
of
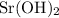 in this solution.
in this solution.
How many moles of
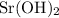 formula units would that be?
formula units would that be?
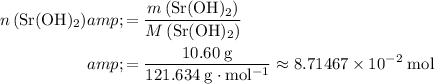 .
.
Molarity of this strontium hydroxide solution
There are
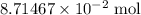 of
of
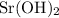 formula units in this
formula units in this
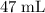 solution. Convert the unit of volume to liter:
solution. Convert the unit of volume to liter:
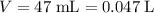 .
.
The molarity of a solution measures its molar concentration. For this solution:
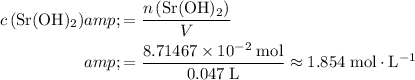 .
.
(Rounded to four significant figures.)