Answer:
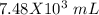
Step-by-step explanation:
For this question we have:
-) A solution NaOH 0.25 M
-) 500 g of glyceryl tripalmitoleate (tripalmitolein)
We can start with the reaction between NaOH and tripalmitolein. NaOH is a base and tripalmitolein is a triglyceride, therefore we will have a saponification reaction. The products of this reaction are glycerol and (E)-hexadec-9-enoate.
Now, with the reaction in mind, we can calculate the moles of NaOH that we need if we use the molar ratio between NaOH and tripalmitolein (3:1) and the molar mass of tripalmitolein (801.3 g/mol). So:
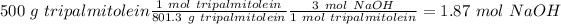
With the moles of NaOH we can calculate the volume (in litters) if we use the molarity equation and the Molarity value:
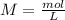
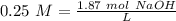
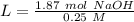
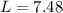
Now we can do the conversion to mL:
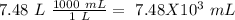
I hope it helps!