Answer:
The volume percent of graphite is 91.906 per cent.
Step-by-step explanation:
The volume percent of graphite (
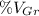 ) is determined by the following expression:
) is determined by the following expression:
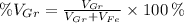
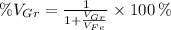
Where:
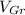 - Volume occupied by the graphite phase, measured in cubic centimeters.
- Volume occupied by the graphite phase, measured in cubic centimeters.
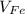 - Volume occupied by the ferrite phase, measured in cubic centimeters.
- Volume occupied by the ferrite phase, measured in cubic centimeters.
The volume of each phase can be calculated in terms of its density and mass. That is:
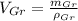
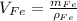
Where:
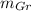 ,
,
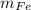 - Masses of the graphite and ferrite phases, measured in grams.
- Masses of the graphite and ferrite phases, measured in grams.
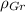 ,
,
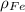 - Densities of the graphite and ferrite phases, measured in grams per cubic centimeter.
- Densities of the graphite and ferrite phases, measured in grams per cubic centimeter.
Let substitute each volume in the definition of the volume percent of graphite:
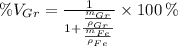
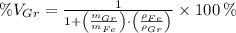
Let suppose that 100 grams of cast iron are available, masses of each phase are now determined:
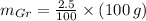
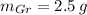
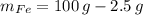
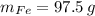
If
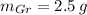 ,
,
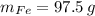 ,
,
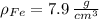 and
and
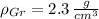 , the volume percent of graphite is:
, the volume percent of graphite is:
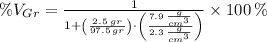
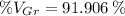
The volume percent of graphite is 91.906 per cent.