Answer:
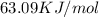
Step-by-step explanation:
In this case, to calculate the heat of solution (KJ/mol) we have to take into account the mass of water, the specific heat of the water and the temperature change, so:
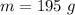
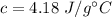
Δ
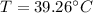
With this in mind, we can use the equation:
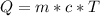
If we plug the values into the equation we will have:
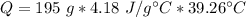
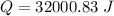
Now, with the mass value (21.5 g) and the molar mass of LiCl (42.39g/mol) we can calculate the moles of LiCl:
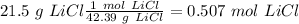
Now, in the heat of solution, we have KJ/mol units. Therefore, we have to convert from J to KJ:
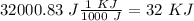
Finally, we can divide by the moles of LiCl:
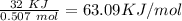
So, for each mole of LiCl, we have 63.09 KJ involved in the dissolution process.
I hope it helps!