Answer:
= 0.2 mL.
Step-by-step explanation:
Given a 0.5 M solution of NaOH as stock solution, 10.0mL of 0.010M can be prepared via dilution with distilled water, by using the formula:
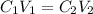
where C1 and V1 are initial concentration and volume respectively; same as C2 & V2 for fina.
Let C1 = 0.5M, V2 = ?
C2 = 0.010M; V2 = 10mL
⇒Volume of stock solution to be diluted, V2
=
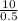 × 0.010
× 0.010
= 0.2 mL.
Glasswares used would be pipette (for smaller volume experiment) and measuring cylinder. 0.2mL would be measured and then made upto the 10mL mark of the measuring cylinder.
I hope this was a detailed explanation given the missing details of "Trial 1" in the question.