Answer:
The pressure the gas will have if the pressure is initially 1.50 atm at 22.0 ° C and the temperature changes at 11.0 ° C is 1.44 atm (option D)
Step-by-step explanation:
Gay Lussac's law indicates that, as long as the volume of the container containing the gas is constant, as the temperature increases, the gas molecules move more rapidly. Then the number of collisions against the walls increases, that is, the pressure increases. That is, the gas pressure is directly proportional to its temperature.
Gay-Lussac's law can be expressed mathematically as follows:
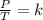
Where P = pressure, T = temperature, K = Constant
You have a gas that is at a pressure P1 and at a temperature T1. When the temperature varies to a new T2 value, then the pressure will change to P2, and then:
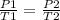
In this case:
- P1= 1.50 atm
- T1= 22 °C= 295 °K (being 0°C= 273 °K)
- P2= ?
- T2= 11 °C= 284 K
Replacing:
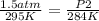
Solving:
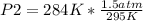
P2=1.44 atm
The pressure the gas will have if the pressure is initially 1.50 atm at 22.0 ° C and the temperature changes at 11.0 ° C is 1.44 atm (option D)