Answer:
24.15%
Step-by-step explanation:
According to the given situation the computation of the percent yield of the reaction is shown below:-
PV = NRT = N =
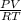
Mole of
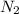 =
=
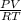
=
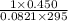
=
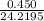
= 0.0186
Mole of
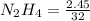
= 0.077
Now, the percentage of yield is
=
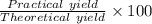
=
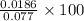
= 24.15%
Therefore for computing the percentage of yield we simply divide the practical yield by theoretical yield and multiply with 100 so that we can get the result into the percentage form.