Answer: The average atomic mass of oxygen is 15.999 amu
Step-by-step explanation:
Mass of isotope O-16 = 15.995 amu
% abundance of isotope O-16= 99.759 % =
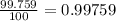
Mass of isotope O-17 = 16.995 amu
% abundance of isotope O-17 = 0.037% =
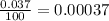
Mass of isotope O-18 = 17.999 amu
% abundance of isotope O-18 = 0.204% =
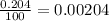
Formula used for average atomic mass of an element :
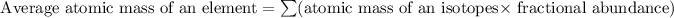
![A=\sum[(15.995* 0.99759)+(16.995* 0.00037)+(17.999 * 0.00204)]](https://img.qammunity.org/2021/formulas/chemistry/college/s3mh87bwphd7mhk2fr284x4i9mk92xc3tc.png)
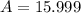
Thus the average atomic mass of oxygen is 15.999 amu