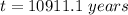Answer:
The painting is
Explanation:
From the question we are told that
The amount of carbon present after t year is
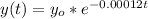 {Note ; This is the function }
{Note ; This is the function }
Here
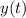 is the amount of carbon-14 after time t
is the amount of carbon-14 after time t
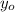 the original amount of carbon-14
the original amount of carbon-14
Now given that the paint as at now contain 27% of the original carbon-14
Then it mean that
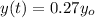
So the equation is represented as
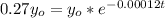
=>
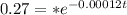
=>
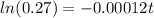
=>
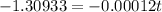
=>
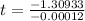
=>