Answer: Thus the cell potential of an electrochemical cell is +0.28 V
Step-by-step explanation:
The calculation of cell potential is done by :
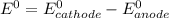
Where both
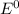 are standard reduction potentials.
are standard reduction potentials.
![E^0_([Fe^(2+)/Fe])= -0.41V](https://img.qammunity.org/2021/formulas/chemistry/college/64ksuzz4dijahp0gafiscoulu9x5jtnglv.png)
![E^0_([Pb^(2+)/Pb])=-0.13V](https://img.qammunity.org/2021/formulas/chemistry/college/hu0kxwtirq0o0w6wjysl2j78dmqc2cldga.png)
As Reduction takes place easily if the standard reduction potential is higher(positive) and oxidation takes place easily if the standard reduction potential is less(more negative). Thus iron acts as anode and lead acts as cathode.
![E^0=E^0_([Pb^(2+)/Pb])- E^0_([Fe^(2+)/Fe])](https://img.qammunity.org/2021/formulas/chemistry/college/4xjim9mfwbjanw4746hdc3yw9q4bxfj23v.png)
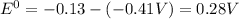
Thus the cell potential of an electrochemical cell is +0.28 V