Answer:
OH⁻ < NH₃ < HCO₃⁻ < H₂O < NH₄⁺ < H₂CO₃
Step-by-step explanation:
We can do some rough calculations to find the approximate pH values of these solutions.
H₂CO₃
Kₐ ≈ 10⁻⁶
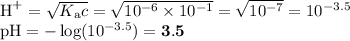
NH₄⁺
Kb of NH₃ ≈ 10⁻⁵
Kₐ of NH₄⁺ ≈ 10⁻⁹
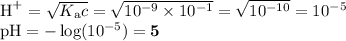
OH⁻
Strong base
[OH⁻] = 10⁻¹
pOH = 1
pH = 14 - 1 = 13
HCO₃⁻
Salt of dibasic acid
K₁ ≈ 10⁻⁶; K₂ ≈ 10⁻¹⁰
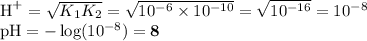
NH₃
Kb ≈ 10⁻⁵
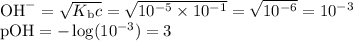
pOH = 14 - 3 = 11
H₂O
Neutral. pH = 7
Order from lowest [H₃O⁺] to highest [H₃O⁺]:
OH⁻ < NH₃ < HCO₃⁻ < H₂O < NH₄⁺ < H₂CO₃
pH 1 3 11 8 7 5 3.5