Answer:
i. Molar mass of glucose = 180 g/mol
ii. Amount of glucose = 0.5 mole
Step-by-step explanation:
The volume of the glucose solution to be prepared = 500
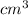
Molarity of the glucose solution to be prepared = 1 M
i. Molar mass of glucose (
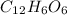 ) = (6 × 12) + (12 × 1) + (6 × 16) = 180 g/mol
) = (6 × 12) + (12 × 1) + (6 × 16) = 180 g/mol
ii. mole = molarity x volume. Hence;
amount (in moles) of the glucose solution to be prepared
= 1 x 500/1000 = 0.5 mole