Answer:
The number of moles of solute present in 4.00 L of an 8.30 M solution is 33.2
Step-by-step explanation:
The Molarity (M) or Molar Concentration is the number of moles of solute per liter of solution; in other words it is the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of the solute by the volume of the solution:
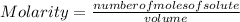
Molarity is expressed in units (
 ) or M.
) or M.
In this case:
- molarity= 8.30 M
- number of moles of solute= ?
- volume= 4.00 L
Replacing:
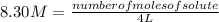
Solving:
number of moles of solute= 8.30 M* 4 L= 8.30
 * 4 L
* 4 L
number of moles of solute =33.2
The number of moles of solute present in 4.00 L of an 8.30 M solution is 33.2