Answer:
rate of recrystallization = 4.99 × 10⁻³ min⁻¹
Step-by-step explanation:
For Avrami equation:
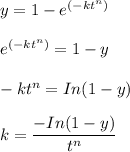
To calculate the value of k which is a dependent variable for the above equation ; we have:
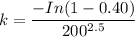
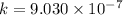
The time needed for 50% transformation can be determined as follows:
![y = 1-e ^((-kt^n)) \\ \\ e^((-kt^n)) = 1-y\\ \\ -kt^n = In(1-y) \\ \\ t =[ (-In(1-y))/(k)]^{^(1/n)}](https://img.qammunity.org/2021/formulas/chemistry/college/ney4k7lcset72iqg9stjeg4ru8sq1kl3mt.png)
![t_(0.5) =[ (-In(1-0.4))/(9.030 * 10^(-7))]^{^(1/2.5)}](https://img.qammunity.org/2021/formulas/chemistry/college/2v0jf4vgciblaedx7dgpsk78c8j3ggc16y.png)
= 200.00183 min
The rate of reaction for Avrami equation is:
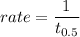
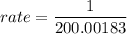
rate = 0.00499 / min
rate of recrystallization = 4.99 × 10⁻³ min⁻¹