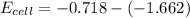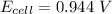Answer:
The voltage is
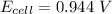
Step-by-step explanation:
Generally the half reaction for Zn, Zn2 half-cell is mathematically represented as
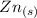 ⇔
⇔
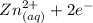 (reference study academy)
(reference study academy)
and the electric potential for this is a constant value
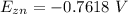
Generally the half reaction for Al, Al3 half-cell is mathematically represented as
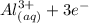 ⇔
⇔
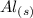
and the electric potential for this is constant value
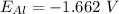
Therefore the cell potential for an electrochemical cell is mathematically represented as
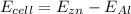
substituting values