Answer:
Step-by-step explanation:
We shall find out volume of air at NTP or at 273 K and 10⁵ Pa ( 1 atm )
Let it be V₂
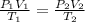
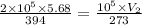
V₂ = 7.87 litres
22.4 litres of any gas is equivalent to 1 mole
7.87 litres of air will be equivalent to
7.87 / 22.4 moles
= .35 moles .