Answer:
The concentration takes 25.360 seconds to decrease to one-ninth of its initial value.
Step-by-step explanation:
The decomposition of the compound has an exponential behavior and process can be represented by this linear first-order differential equation:
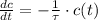
Where:
 - Time constant, measured in seconds.
- Time constant, measured in seconds.
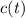 - Concentration of the compound as a function of time.
- Concentration of the compound as a function of time.
The solution of the differential equation is:
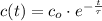
Where
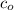 is the initial concentration of the compound.
is the initial concentration of the compound.
The time is now cleared in the result obtained previously:
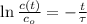
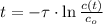
Time constant as a function of half-life is:
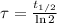
Where
 is the half-life of the composite decomposition, measured in seconds.
is the half-life of the composite decomposition, measured in seconds.
If
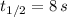 , then:
, then:
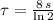
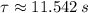
And lastly, given that
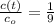 and
and
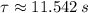 , the time taken for the concentration to decrease to one-ninth of its initial value is:
, the time taken for the concentration to decrease to one-ninth of its initial value is:
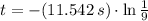
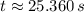
The concentration takes 25.360 seconds to decrease to one-ninth of its initial value.