Answer: The energy of a Br–F bond is 110 kJ/mol
Step-by-step explanation:
The balanced chemical reaction is,
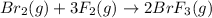
The expression for enthalpy change is,
![\Delta H=\sum [n* B.E(reactant)]-\sum [n* B.E(product)]](https://img.qammunity.org/2021/formulas/chemistry/college/pzc2s7knssmaplhmlikv472zh38zvjrmfy.png)
![\Delta H=[(n_(Br_2)* B.E_(Br_2))+(n_(F_2)* B.E_(F_2)) ]-[(n_(BrF_3)* B.E_(BrF_3))]](https://img.qammunity.org/2021/formulas/chemistry/college/etxktu47j7r0bur7budxrz0o2g9okuk05z.png)
![\Delta H=[(n_(Br_2)* B.E_(Br-Br))+(n_(F_2)* B.E_(F_F)) ]-[(n_(BrF_3)* 3* B.E_(Br-F))]](https://img.qammunity.org/2021/formulas/chemistry/college/pxxatb70cyu5r5557z3t1sieb82pqrtoeo.png)
where,
n = number of moles
Now put all the given values in this expression, we get
![\Delta H=[(1* 193)+(3* 155)]-[(2* 3* B.E_(Br-F))]](https://img.qammunity.org/2021/formulas/chemistry/college/6fyfs14vqpgdz8x5ae7f30pxy15nj6lyk0.png)
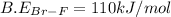
Thus the energy, in kJ/mol, of a Br–F bond is 110