Answer:
35%
Step-by-step explanation:
Percentage yield = actual yield / theoretical yield × 100.
Given:
Actual yield = 63.07g
Theoretical yield = ?
Mole ratio of C7H6O3 to C4H6O3 = 1 : 1
1 mole of C7H6O3 - 138.1g
Which implies that only 1 mole s
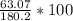 hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g
hould be used up in the reaction, yielding 180.2 g of C9H8O4. ⇒ Theoretical yield = 180.2g
∴ % Yield =
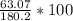
= 35% yield.
Let me know if you found this easy to understand.