Answer:
The values of a, b, c and d are
a = 3, b = 3, c = 4 and d = 3
Step-by-step explanation:
The quantity of heat needed to melt the ice is given by Q = mL where m = mass of ice and L = latent heat of fusion of ice = 334 kJ/kg. This quanity of heat is also the thermal energy needed to be released by the calcium chloride, CaCl₂
Now, the mass of ice = 10 kg. So,
Q = mL
Q = 10 kg × 334 kJ/kg
Q = 3340 kJ
In scientific notation,
Q = 3.34 × 10³ kJ
So the thermal energy needed to be released by the calcium chloride is 3.34 × 10³ kJ
Comparing Q = 3.34 × 10³ kJ with a.bc ×
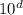 kJ
kJ
So, a = 3, b = 3, c = 4 and d = 3
The values of a, b, c and d are
a = 3, b = 3, c = 4 and d = 3