Answer:
0.35 V
Step-by-step explanation:
(a) Standard reduction potentials
E°/V
Fe²⁺ + 2e- ⇌ Fe; -0.41
Cr³⁺ + 3e⁻ ⇌ Cr; -0.74
(b) Standard cell potential
E°/V
2Cr³⁺ + 6e⁻ ⇌ 2Cr; +0.74
3Fe ⇌ 3Fe²⁺ + 6e-; -0.41
2Cr³⁺ + 3Fe ⇌ 2Cr + 3Fe²⁺; +0.33
3. Cell potential
2Cr³⁺(0.75 mol·L⁻¹) + 6e⁻ ⇌ 2Cr
3Fe ⇌ 3Fe²⁺(0.25 mol·L⁻¹) + 6e-
2Cr³⁺(0.75 mol·L⁻¹) + 3Fe ⇌ 2Cr + 3Fe²⁺(0.25 mol·L⁻¹)
The concentrations are not 1 mol·L⁻¹, so we must use the Nernst equation
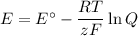
(a) Data
E° = 0.33 V
R = 8.314 J·K⁻¹mol⁻¹
T = 298 K
z = 6
F = 96 485 C/mol
(b) Calculations:
![Q = \frac{\text{[Fe}^(2+)]^(3)}{ \text{[Cr}^(3+)]^(2)} = (0.25^(3))/( 0.75^(2)) =(0.0156)/(0.562) = 0.0278\\\\E = 0.33 - \left ((8.314 * 298)/(6 * 96485)\right ) \ln(0.0278)\\\\=0.33 -0.00428 * (-3.58) = 0.33 + 0.0153 = \textbf{0.35 V}\\\text{The cell potential is }\large\boxed{\textbf{0.35 V}}](https://img.qammunity.org/2021/formulas/chemistry/college/aby47vvpfdffu7ql46hvxtlgyuonfthe43.png)