Answer:
F = 57.6 N
Step-by-step explanation:
An electrostatic force is either a force of attraction or repulsion between two charges. When the two charges are like charges, the force is that of repulsion. But when they are of opposite charges, then the force between them is an attractive force.
A proton has a charge of 1.6 ×
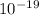 C. The electrostatic force can be determined by;
C. The electrostatic force can be determined by;
F =
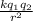
Where: k is a constant,
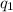 is the first charge,
is the first charge,
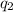 is the second charge, and r is the distance between the charges.
is the second charge, and r is the distance between the charges.
But,
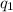 =
=
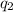 = 1.6 ×
= 1.6 ×
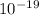 C, k = 9 ×
C, k = 9 ×
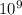 N
N
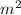 /
/
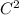 , r = 2 ×
, r = 2 ×
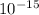 m. Therefore;
m. Therefore;
F =
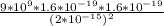
=
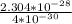
= 57.6 N
The electrostatic force between the protons is 57.6 N.