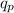Answer:
ΔH =
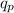
Step-by-step explanation:
In a calorimeter, when there is a complete combustion within the calorimeter, the heat given off in the combustion is used to raise the thermal energy of the water and the calorimeter.
The heat transfer is represented by
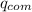 =
=
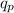
where
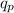 = the internal heat gained by the whole calorimeter mass system, which is the water, as well as the calorimeter itself.
= the internal heat gained by the whole calorimeter mass system, which is the water, as well as the calorimeter itself.
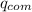 = the heat of combustion
= the heat of combustion
Also, we know that the total heat change of the any system is
ΔH = ΔQ + ΔW
where
ΔH = the total heat absorbed by the system
ΔQ = the internal heat absorbed by the system which in this case is
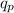
ΔW = work done on the system due to a change in volume. Since the volume of the calorimeter system does not change, then ΔW = 0
substituting into the heat change equation
ΔH =
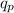 + 0
+ 0
==> ΔH =