Answer:
19.07 g mol^-1
Step-by-step explanation:
The computation of the molecular mass of the unknown gas is shown below:
As we know that
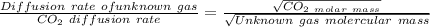
where,
Diffusion rate of unknown gas = 155 mL/s
CO_2 diffusion rate = 102 mL/s
CO_2 molar mass = 44 g mol^-1
Unknown gas molercualr mass = M_unknown
Now placing these values to the above formula
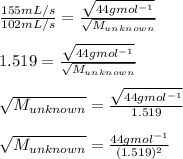
After solving this, the molecular mass of the unknown gas is
= 19.07 g mol^-1