Answer:
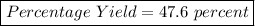
Step-by-step explanation:
The balanced chemical reaction is
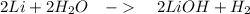
For the balanced reaction, we've
Lithium = 14 g
Water = 36 g
LiOH = 48 g
Hydrogen gas = 2 g
So,
14 g of lithium = 48 g of lithium hydroxide
And we have:
7.40 g of lithium = x grams of lithium hydroxide
Cross Multiplying,
x grams =
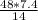
Grams of lithium = 25.4 g of lithium hydroxide
So, Theoretical Yield = 25.4 g of lithium hydroxide
Now, The %age yield:
%age Yield =
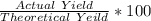
%age Yield =
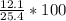
%age Yield = 47.6%