Answer:
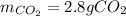
Step-by-step explanation:
Hello,
In this case, the combustion of octane is chemically expressed by:
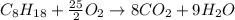
In such a way, due to the 25/2:8 molar ratio between oxygen and carbon dioxide, we can compute the yielded grams of carbon dioxide (molar mass 44 g/mol) as shown below:
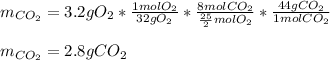
Best regards.