Answer:

The reactant that is reduced is Q.
Step-by-step explanation:
The complete equation for the reaction is such that:

Two molecules of H atom is lost from
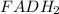 and the H atoms are gained by the coenzyme Q. Consequently,
and the H atoms are gained by the coenzyme Q. Consequently,
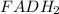 becomes FAD while Q becomes
becomes FAD while Q becomes
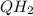 .
.
From the definition of oxidation as loss of hydrogen and reduction as the addition of hydrogen, it can be concluded that the FADH2 that lost hydrogen is a reactant that is oxidized while the coenzyme Q that gained hydrogen is a reactant that is reduced in the reaction.