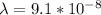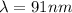Answer:
The wavelength is
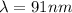
Step-by-step explanation:
From the question we are told that
The energy required is
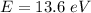
This energy needed in form of a photon can be mathematically represented as
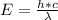
where h is the Planck constant with a value
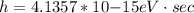
and c is the speed of light which is
substituting values
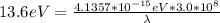
= >