Answer: The equilibrium value of
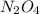 is 0.379 M
is 0.379 M
Step-by-step explanation:
Equilibrium constant is the ratio of the concentration of products to the concentration of reactants each term raised to its stochiometric coefficients.
Using ideal gas equation :

P = pressure of gas
V = volume of gas
n = no of moles
R = gas constant
T = Temperature
pressure of
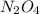 =
=
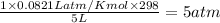
pressure of
 =
=
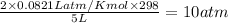
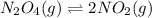
at t= 0 5 atm 10 atm
at eqm (5-x) atm (10+2x) atm
![K_p=([p_NO_2]^2)/([p_N_2O_4])](https://img.qammunity.org/2021/formulas/chemistry/college/2lvqtaxi1377jf6a0csn70xegtxnmmbzyf.png)
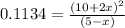
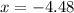
pressure of
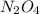 at equilibrium = (5-(-4.48))= 9.48 atm
at equilibrium = (5-(-4.48))= 9.48 atm
pressure of
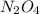 =
=
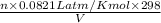
9.48 =
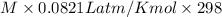
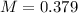
Thus the equilibrium value of
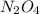 is 0.379 M
is 0.379 M