Answer:
A)
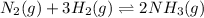 .
.
B)
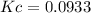 .
.
C) 0.9 mol.
D) Increasing both temperature and pressure.
Step-by-step explanation:
Hello,
In this case, given the information, we proceed as follows:
A)
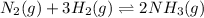
B) For the calculation of Kc, we rate the equilibrium expression:
![Kc=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/college/3ifkn5oa5thlyvhg2voh7us1v47g9hwnmb.png)
Next, since at equilibrium the concentration of ammonia is 0.6 M (0.9 mol in 1.5 dm³ or L), in terms of the reaction extent
 , we have:
, we have:
![[NH_3]=0.6M=2*x](https://img.qammunity.org/2021/formulas/chemistry/college/7ecnd3f08i92l45wun7lf6cs8h9pjsfuh5.png)
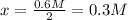
Next, the concentrations of nitrogen and hydrogen at equilibrium are:
![[N_2]=(1.5mol)/(1.5L)-x=1M-0.3M=0.7M](https://img.qammunity.org/2021/formulas/chemistry/college/l159j3msryf1xxdvdlh011v5mkhe6kzhw5.png)
![[H_2]=(4mol)/(1.5L)-3*x=2.67M-0.9M=1.77M](https://img.qammunity.org/2021/formulas/chemistry/college/ng2wu4ukak0hd94gmuju9hcjwhc5af0ni8.png)
Therefore, the equilibrium constant is:
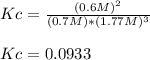
C) In this case, the equilibrium yield of ammonia is clearly 0.9 mol since is the yielded amount once equilibrium is established.
D) Here, since the reaction is endothermic (positive enthalpy change), one way to increase the yield of ammonia is increasing the temperature since heat is reactant for endothermic reactions. Moreover, since this reaction has less moles at the products, another way to increase the yield is increasing the pressure since when pressure is increased the side with fewer moles is favored.
Best regards.