Answer:
1,4-hexanediamine contains two
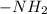 functional groups.
functional groups.
Step-by-step explanation:
1,4-hexanediamine is an organic molecule which contains two
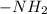 functional groups at C-1 and C-4 position.
functional groups at C-1 and C-4 position.
The longest carbon chain in 1,4-hexanediamine contains six carbon atoms.
Molecular formula of 1,4-hexanediamine is
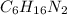 .
.
1,4-hexanediamine used as a bidentate ligand in organometallic chemistry.
The structure of 1,4-hexanediamine is shown below.