Answer:
b) rate = kAB.
Step-by-step explanation:
Hello,
In this case, considering the given statement, we can notice that the rate law of the overall reaction will be determined for the slowest step, that is:
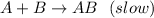
In such a way, we can infer that the rate law will contain both the concentration of A and B to the first power both, since their stoichiometric coefficients in the chemical equation are both one:
![rate=k[A][B]](https://img.qammunity.org/2021/formulas/chemistry/college/k3iy1ljn19aqzb1phkj91sv7paifujxic1.png)
Thereby, answer is b) rate = kAB, that should be better rate = k[A][B] by expressing the concentrations.
Best regards.