Answer:
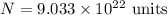
Step-by-step explanation:
It is given that,
Number of moles, n = 0.15
We need to find the number of particles in 0.15 mol of NaCl. Let N are the number of particles i.e.
Number of particles = number of moles × Avagadro's number
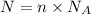
So,
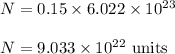
So, the number of particles are
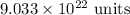 .
.