Answer:
P SO₂ = 0.06atm
P O₂ = 2.635atm
P SO₃ = 0.53atm
Kp = 29.6
Step-by-step explanation:
The reaction of Sulfur dioxide and oxygen react to form sulfur trioxide is as follows:
2SO₂(g) + O₂(g) ⇄ 2SO₃(g)
And Kp is defined as:
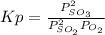
Where P represents the pressure at equilibrium of each reactant.
If you add, in the first, 0.59atm of SO₂ and 2.9atm of O₂, the equilibrium pressures will be:
P SO₂ = 0.59atm - 2X
P O₂ = 2.9atm - X
P SO₃ = 2X
Where X represents the reaction coordiante.
As equilibrium pressure of SO₃ is 0.53atm:
0.53atm = 2X
0.265atm = X
Replacing, equilibrium pressures of each species will be:
P SO₂ = 0.59atm - 2×0.265atm
P O₂ = 2.9atm - 0.265atm
P SO₃ = 2×0.265atm
P SO₂ = 0.06atm
P O₂ = 2.635atm
P SO₃ = 0.53atm
And Kp will be:
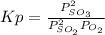

Kp = 29.6