Answer: 38.52 moles of hydrogen will be required to produce 19.26 mol of methane
Step-by-step explanation:
To calculate the moles :
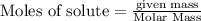
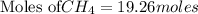
The balanced chemical reaction is:
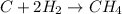
According to stoichiometry :
1 mole of
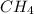 is produced by = 2 moles of
is produced by = 2 moles of
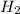
Thus 19.26 moles of
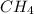 will be produced by =
will be produced by =
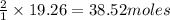 of
of
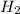
Thus 38.52 moles of hydrogen will be required to produce 19.26 mol of methane