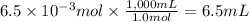Answer:
6.5 mL
Step-by-step explanation:
Step 1: Write the balanced reaction
Ca(OH)₂ + 2 HNO₃ ⇒ Ca(NO₃)₂ + 2 H₂O
Step 2: Calculate the reacting moles of nitric acid
25.0 mL of 0.50 M nitric acid react.
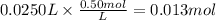
Step 3: Calculate the reacting moles of calcium hydroxide
The molar ratio of Ca(OH)₂ to HNO₃ is 1:2. The reacting moles of Ca(OH)₂ are 1/2 × 0.013 mol = 6.5 × 10⁻³ mol
Step 4: Calculate the volume of calcium hydroxide
To answer this, we need the concentration of calcium hydroxide. Since the data is missing, let's suppose it is 1.0 M.