Answer:
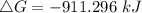
Step-by-step explanation:
ΔG = ΔH-TΔS
Where ΔH = -176 kJ = -176000 J , T = 25°C + 273 = 298 K , ΔS = -284.8 JK⁻¹
=>
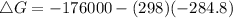
=>
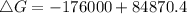
=>
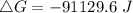
=>
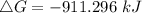
Since the value is negative, the reaction is spontaneous under standard conditions at 298 K and the reactants have more free energy than the products.