Answer: The concentration of the mixture is 0.5 p % .
Explanation:
Given: 5 gallons of p% boric acid is mixed with 5 gallons of water.
Amount of boric acid = p% of 5 gallons
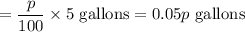
Total solution : 5 +5 = 10 gallons
then, the concentration of the mixture =
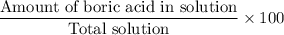
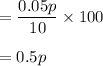
Hence, the concentration of the mixture is 0.5 p % .