Answer:
It will take 18,569.2 years for carbon–14 to decay to 10 percent of its original amount
Explanation:
The amount of Carbon-14 after t years is given by the following equation:
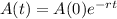
In which A(0) is the initial amount and r is the decay rate, as a decimal.
Carbon–14 is a radioactive isotope that decays exponentially at a rate of 0.0124 percent a year.
This means that
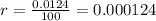
How many years will it take for carbon–14 to decay to 10 percent of its original amount?
This is t for which:
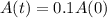
So
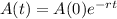
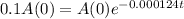
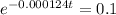
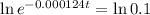
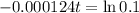
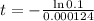
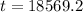
It will take 18,569.2 years for carbon–14 to decay to 10 percent of its original amount