Answer: The percentage composition of nitrogen , hydrogen and oxygen is 22.2 % , 1.59 % and 76.2% respectively.
Step-by-step explanation:
Percentage composition is defined as the ratio of mass of substance to the total mass in terms of percentage.
Percentage composition=
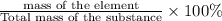
a)
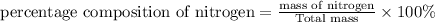
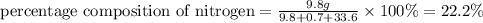
b)
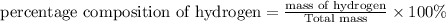
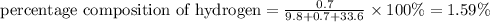
c)
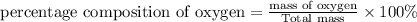
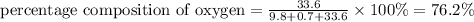
The percentage composition of nitrogen , hydrogen and oxygen is 22.2 % , 1.59 % and 76.2% respectively.