Answer:
C. 1.07 M.
Step-by-step explanation:
Hello,
In this case, we can define the molarity of the bleach as shown below:
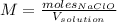
In such a way, given the mass of bleach in a 1-L solution, we can compute the density:
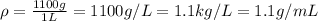
In such a way, we can use the previously computed density to compute the volume of the solution, assuming a 100-g solution given the by-mass percent:
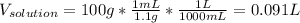
Afterwards, using the by-mass percent of bleach we compute the mass:
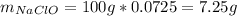
And the moles:
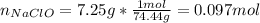
Therefore, the molarity turns out:
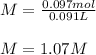
Thus, answer is C. 1.07 M.
Regards.