Answer:
C.
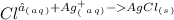
Step-by-step explanation:
In this question our options are:
A.
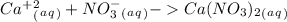
B.
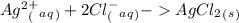
C.
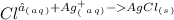
D. None of the above because no reaction occurs
We have to remember that the ions produced by
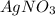 are:
are:
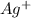 and
and
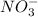
And the ions produced by
 are:
are:
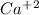 and
and

Additionally, we will have a double displacement reaction so the compounds produce are:
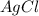 and
and
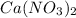
If we remember the solubility rules, all the nitrate salts are soluble and the salts made with silver are not soluble. With this in mind, we will have a solid-state for
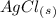 and an aqueous state for
and an aqueous state for
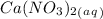 .
.
If this is true, the final answer can be B or C. The charge of Ag is +1 so the final answer is C.
I hope it helps!