Answer:
See explanation
Step-by-step explanation:
In this case, we have to keep in mind the valence electrons for each atom:
N => 5 electrons
O => 6 electrons
If the formula is
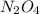 , we will have in total:
, we will have in total:
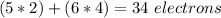
Additionally, we have to remember that each atom must have 8 electrons. So, for oxygens 5 and 3 we will have 3 lone pairs and 1 bond (in total 8 electrons. For oxygens, 6 and 4 we will have 2 lone pairs and 2 bonds (in total 8 electrons) and for nitrogens 1 and 2 we will have 4 bonds (in total 8 electrons).
To find the hybridization, we have to count the atoms and the lone pairs around the nitrogen. We have 3 atoms and zero lone pairs. If we take into account the following rules:
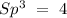
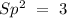
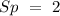
With this in mind, the hybridization of nitrogen is
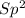 .
.
See figure 1
I hope it helps!