Answer:
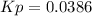
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
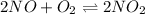
For which the equilibrium expression is:
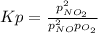
Whereas, at equilibrium, each pressure is computed in terms of the initial pressure and the reaction extent via:
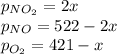
And the total pressure:
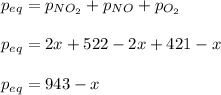
Yet it is 748 torr, for which the extent is:
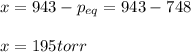
Therefore, Kp turns out:
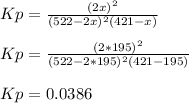
Best regards.