Answer:
The answer is "At 2200K, NO(g)'s partial pressure is below 2500K. C.KP by a factor of (RT) is lower than Kc"
Step-by-step explanation:
In the given question choices were missing so, the correct choice can be defined as follows:
Given:
Reaction equation:
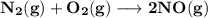
when temperature 2,200 K the value of KP =
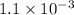
when temperature 2,500 K the value of KP =
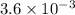
value of kp=?
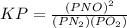
In the above formula the value of
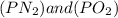 is defined in the denominator section and the value of
is defined in the denominator section and the value of
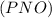 is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).
is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).