Answer:
E. 8.08 x 10⁴.
Step-by-step explanation:
Hello,
In this case, for the reaction:
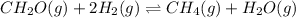
We can compute the Gibbs free energy of reaction via:
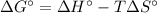
Since both the entropy and enthalpy of reaction are given at 298 K (standard temperature), therefore:
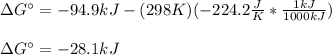
Then, as the equilibrium constant is computed as:
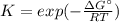
We obtain:
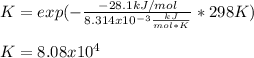
For which the answer is E. 8.08 x 10⁴.
Best regards,