Answer:
3-methylpentan-3-ol
Step-by-step explanation:
In this case, we have an "Oxymercuration reaction". With this in mind, we will have to add an "OH" to the most substituted carbon of the double bond and we will obtain 3-methylpentan-3-ol. To understand how this molecule is produced we have to check the mechanism:
The mercury(II) acetate (
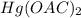 ) is an ionic substance. So, this substance can be ionized into his ions and we will have the cation
) is an ionic substance. So, this substance can be ionized into his ions and we will have the cation
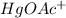 and the anion
and the anion
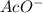 . The cation will attack the double bond and vice-versa to produce a "cyclic intermediate". Then a water molecule will attack the most substituted carbon and the cyclic compound would be broken producing a new bond C-O with a positive charge in the oxygen. Then a deprotonation step takes place and finally, the
. The cation will attack the double bond and vice-versa to produce a "cyclic intermediate". Then a water molecule will attack the most substituted carbon and the cyclic compound would be broken producing a new bond C-O with a positive charge in the oxygen. Then a deprotonation step takes place and finally, the
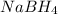 would reduce the compound to produce the final alcohol.
would reduce the compound to produce the final alcohol.
See figure 1
I hope it helps!